What are Counterfeit Medicines?
Counterfeit medicines are made to mimic their authentic counterparts with the intention to mislead consumers. They are sold under a product name without proper authorisation. Counterfeiting can apply to both brand name and generic products. Counterfeit medicines may include products without the active ingredient, with an insufficient or excessive quantity of the active ingredient, with the wrong active ingredient, or with fake packaging.1
Counterfeiting is generally difficult to detect, investigate, and quantify. It is hence not surprising that the full extent of the problem is not well understood. According to the World Health Organisation (“WHO”), the global market for counterfeit medicines is estimated to be worth between US$75 and US$200 billion. These counterfeit medicines are distributed to both developed and developing nations via complex global networks that have been traced to crime syndicates and terrorists.
Why is Counterfeit Medicine a Problem?
Counterfeit medicine is a growing global problem particularly in developing nations where legitimate medicines are beyond the reach of poor or low income families. These medicines which may be manufactured in small backstreet operations under poor conditions and lack of proper quality control are usually sub-standard and cheaper than legitimate medicines. Additionally, these countries have weak or no regulation system to prevent, deter or curb the proliferation of counterfeit medicines in the market, even in legitimate institutions such as public hospitals.

Counterfeit medicines may not always result in obvious adverse reactions though they may fail to treat the conditions they were intended for. In the documentary film “Pharmacide Mekong”, Dr Yim Yann, Undersecretary of State, Ministry of Health, Cambodia lamented “I feel a lot of regret for people who got sick and could not be cured. To recover from malaria would have cost not more than 10 dollars. So, how could they die? We have enough anti-malarial drugs. Why did these people have access only to fake drugs and not to quality drugs? Was the death of hundreds of people caused by their destiny or was it a failure of our system?”
In developed countries, the culture of self-diagnosis and self-prescription coupled with the increased use of the internet to purchase various types of drugs have “assisted” counterfeiters to penetrate the market as the internet is rarely subjected to effective oversight and control.
Beyond merely the infringement of intellectual property rights, these poor quality medicines can result in the decreased efficacy of medicine in combating diseases, increased drug resistance, increased mortality and morbidity, adverse effects from incorrect active ingredients (eg, toxic heavy metals), loss of confidence in the health systems and workers, economic loss for patients, families, drug producers, and wastage of resources in the development of new medicines.
What is the Purpose of Analysis?
Given the significant danger that counterfeit medicines pose to public health, combating counterfeit medicines requires the on-going cooperation of numerous stakeholders including regulatory authorities, law enforcement officers, pharmaceutical companies, healthcare providers and patients.
Products suspected to be counterfeit medicines are seized by regulatory authorities and law enforcement officers during pre- and post-market surveillance and during investigative operations. Detection of counterfeits in the legal supply chain triggers product recall. These products will be examined to:
1. identify the ingredients in the medicines (presence and amount of active pharmaceutical ingredients, contaminants etc),
2. determine the product’s authenticity,
3. determine whether the counterfeit products could have originated from the same source.
In cases of product recalls, the regulatory authority will notify the supplier of the product and the manufacturer may be involved in the investigation. Although pharmaceutical companies may have their own testing laboratories to carry out the analysis of the spurious medicines, such examinations and testing are best carried out by an independent forensic laboratory to avoid issues related to conflict of interest.
Types of Counterfeit Medicine
There is a wide array of counterfeit medicines as counterfeiters target drugs in different therapeutic categories. According to the Pharmaceutical Security Institute (“PSI”),2 medications for chronic conditions are most popular, e.g. hypertensive drugs and diabetes medicines. Antibiotics,3 antimalarials,3 corticosteroids, drugs for erectile dysfunction, cancer drugs and antiretrovirals for HIV/AIDS are also among those most counterfeited.
In the past, counterfeiters manufacture products which mimicked the authentic products in their appearance. Most of these products did not contain any active pharmaceutical ingredient (“API”). However, the counterfeiters of modern times have grown in business sense. Instead of producing medicines which do not have any therapeutic value, they are looking for repeat business. APIs are cheap and readily available. Hence, many of the counterfeit medicines we see today contain APIs; some with similar amounts of APIs as the authentic medicine, and others with higher quantities of APIs than the authentic medicine, with the intention to “increase” the therapeutic effects.
Just like counterfeiting of currency, counterfeiters of medicines also make use of advancements in technology to create “super-counterfeits”. These are high quality counterfeits which are very difficult to differentiate from their authentic products by the naked eye or using portable devices. Many of the cases encountered involved a counterfeit cartons and blister packs that appear more pristine than the authentic product in their materials and print. Even security features such as holograms or colour-shifting inks which were once effective anti-counterfeiting measures have been effectively “copied”.
Detection of Counterfeit Medicine
Drug manufacturers and distributors integrate security features into their products to ensure product integrity and supply chain integrity. These security features generally pertain to the packaging of the medicine. Although some of these features were introduced to enable the consumer to differentiate counterfeits from authentic products, not all proved to be effective as the consumer was unable to differentiate between a real and a fake security feature. Another interesting point to highlight is that although authentication of packaging materials is easier to perform than chemical analysis, it cannot conclusively lead to the claim of authenticity for the drug contained within the packaging. Ironically, one needs to remember that it is the drug that affects a person’s well-being, not the packaging.
Physical examination: Security features may be overt or covert.
1. Overt features such as the use of colour-shifting inks on packaging allow quick and easy authentication of a product by human senses without the use of instruments. Other overt features such as holograms and watermarks may require the use of portable devices or external stimulation (e.g., ultra-violet light) for authentication.
2. Covert features such as microprinting and use of taggants can only be verified using portable devices or some level of chemical analysis.
Chemical Analysis: On-site portable devices are used to screen seized products for the presence of the APIs stated on the product labels. Such hand-held instruments are easy to use and require little or no sample preparation. They are useful as a screening tool, to quickly sieve out the potential counterfeits and assist the investigator to effectively sample the seized products for laboratory analysis. The identities of the detected APIs are confirmed by analytical instruments in the laboratory. These instruments will also quantify the APIs, based on pharmaceutical monographs, to determine whether the APIs are present in the correct quantities. The above-mentioned methodology used to be the protocol for detecting counterfeit medicines. With the advent of “super-counterfeits”, a multi-disciplinary approach based on a combination of complementary techniques is required for the effective detection of counterfeit medicines and counterfeit APIs.
Counterfeit APIs are more difficult to detect than counterfeit medicines. In counterfeit medicines, forensic scientists examine the overall chemical fingerprint of the different ingredients (excipients, APIs and impurities) present in the medicine. For counterfeit APIs, scientists target the impurities and residual solvents which are present in trace amounts.
In 2007, our senior forensic experts were part of a team to develop a multi-disciplinary approach4–7 for the analysis of counterfeit medicines and APIs in Singapore. This holistic assessment integrates data and findings from both physical and chemical techniques. See Figure 1. Additionally, the physical and chemical information obtained was used to build a counterfeit drugs database which allowed the following activities to be performed:
1. Monitoring of counterfeit drugs trends;
2. Identification of counterfeit drugs by searching the database using the input information;
3. Provision of investigative leads through linkages established between different seizures, especially those from different locations and geographical origins; and
4. Sharing of information with overseas counterparts.
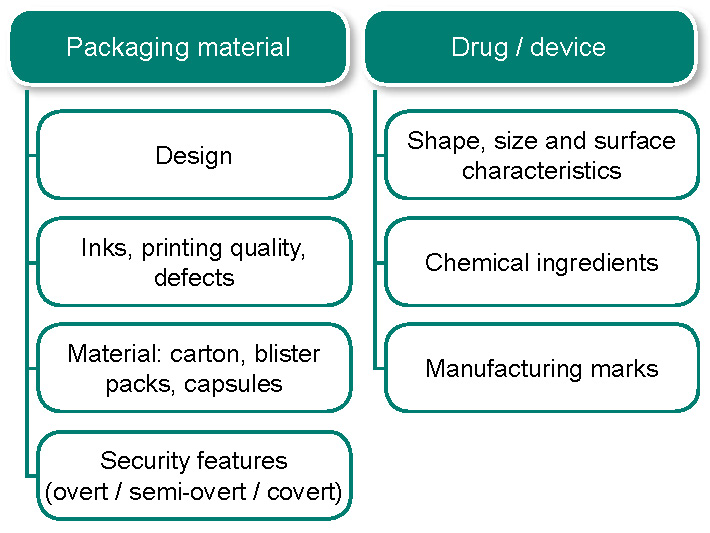
Figure 1: Multi-disciplinary approach to the analysis of counterfeit products
Challenges in the Analysis of Counterfeit Medicines
The analysis of counterfeit medicines requires the expertise of a multi-disciplinary scientist with a strong background in forensic marks examination, document examination and analysis of unknown chemicals and materials. Expert knowledge and experience in forensic marks and document examination allow the scientist to recognise important security features on the packaging, evidence of tampering and significant manufacturing marks that may not be apparent to other scientists. This enables the forensic scientist to not only differentiate the counterfeit products from the authentic products, but also provide important investigative leads on potential common origins between counterfeit products.
Similar to the analysis of unknown chemicals and materials (“unknowns”), the scientist must ensure that the techniques employed are scientifically sound and appropriately validated. It is vital for the scientist to understand the analytical limitations of each method selected, as different combinations of techniques are required for different products.8
Some of the challenges encountered in the analysis of unknowns also apply to the analysis of counterfeit medicines:
1. Availability of authentic samples from the manufacturer for comparison.
2. Availability and cost of purchasing pure APIs as standards for qualitative and quantitative analysis.
3. Adulterants or contaminants are often present in trace amounts, adding to the difficulty in their isolation and detection.
To surmount the above challenges, drug manufacturers, distributors and independent laboratories must work together to share information through collaborative research into new detection technologies.
Local Cases Involving Counterfeit Medicines and Medical Devices
Most of the counterfeit drugs encountered in Singapore are mainly lifestyle drugs, e.g. erectile dysfunction drugs purchased from dubious sources. Some of the local cases of counterfeit medicines and medical devices which our experts were involved in are highlighted below.
Wrong Active Pharmaceutical Ingredient in Sexual-enhancement Products9
In 2012, four men, aged between 30 and 78, were hospitalised with dangerously low blood-sugar levels after taking illegal sexual-enhancement products. Besides the API Sildenafil which is commonly found in sexual-enhancement products, Glibenclamide, a prescription drug for diabetes, was found in the urine and blood of the four men and identified as the cause of their dangerously low blood-sugar levels.
Interestingly, Glibenclamide was found in more than one type of illegal sexual-enhancement products. In one of the products, the amount of Glibenclamide was about five times the usual amount prescribed to a diabetic patient, while the amount of Sildenafil was far below the 25 mg needed to trigger a sexual enhancement effect.
The use of Glibenclamide by the counterfeiters remains a mystery but the presence of this ingredient facilitated the tracing of seized products from different geographical locations to a common source.
Generally, although consumers are aware that drugs purchased from backstreet “pharmacies” and online peddlers have caused fatalities previously, and there are no guarantees that such drugs are bona fide, they still fall prey to counterfeit medicines. They are usually lured by the quick-fixes these medicines provided, and the low cost of the drugs which can be as little as $1, compared to authentic drugs which cost about $20 a pill.
Counterfeit Contact Lenses (Public Prosecutor v Koh Peng Kiat)10, 11
CIBA VISION was first alerted to the counterfeit contact lenses being sold in optical shops in Singapore when it received stocks of “FreshLooks®ColorBlends®” contact lenses for exchange from several optical shops. Laboratory examination of the suspicious products found that their packaging and chemical ingredients differed from those of the authentic products. They were unsafe, of poor quality and deficient in many aspects. The laboratory findings provided strong support to the investigations.
INTERPOL-led Operation Storm II12
A multi-country police operation targeting the manufacture and distribution of counterfeit medicines in Southeast Asia resulted in more than 30 arrests and the seizure of 20 million fake and illegal medicines. It also led to the closure of more than 100 pharmacies and illicit drug outlets. Singapore, and another laboratory from the United States, were designated to provide the forensic support during the operation. The success of Operation Storm demonstrated that collaboration involving public and private sector partnerships and joint international action is crucial to significantly disrupt the trade in counterfeit medicines.
Conclusion
Economic incentives provided by the high demand for drugs, ease of manufacturing counterfeit products, and the ability to sell drugs directly to consumers without face-to-face contact through the internet has escalated the growth of counterfeit medicines globally. It is a menace which appears to be difficult to eradicate and increasingly on the rise. The significant threat to public health, huge economic losses to drug manufacturers and heavy burden imposed on regulatory authorities, law enforcement agencies and healthcare providers necessitates the multi-national and multi-agency collaboration between both the public and private sectors to combat against this global crime. Forensic laboratories with multi-disciplinary capabilities play a key role in supporting the analysis and identification of these counterfeit products, and tracing them to a common source.
The Forensic Experts Group*
E-mail: [email protected]
* The Forensic Experts Group (“TFEG”) is Singapore’s first one-stop private and independent provider of forensic consultancy, analysis, research, training and education for legal and law enforcement agencies, forensic and tertiary institutions, and private organisations. It comprises a team of accomplished and innovative forensic scientists, who are combining 75 years of specialised knowledge, unique experience and skillsets to deliver high quality forensic services both locally and overseas. While leading the Criminalistics Laboratory and the Forensic Chemistry & Physics Laboratory at the Health Sciences Authority from the mid-1990s, TFEG’s forensic scientists developed methods and analytical schemes, and conducted analyses of counterfeit medicines and other products in Singapore.
www.forensicexperts.com.sg
Notes
1 http://www.fda.gov/Drugs/DrugSafety/ucm169898.htm.
2 http://www.fiercepharma.com/special-report/top-counterfeit-drugs-report.
3 Yong Yuk Lin, Plancon A, Lau Yen Hui, Hostetler DM, Fernandez FM, Green DG, Sounvoravong S, Nara S, Boravann M, Dumrong T, Bangsawan N, Tuc VV, Low Min Yong, Lim Chin Chin, Lee Ruth, Paul Newton, “Quality of Antimalarials and Antibiotics in SE Asia. Collaborative Health and Enforcement Operations on the Quality of Antimalarials and Antibiotics in SE Asia”, American Journal of Tropical Medicine & Hygiene, April 2015.
4 Lim Chin Chin, Yong Yuk Lin, Yang Chiew Yung, “Detection of Counterfeit Drugs – Singapore’s Approach” (2011), APEC Life Science Innovation Forum First Anti-Counterfeiting Health Products Seminar, Beijing, China.
5 Lim Chin Chin, Yong Yuk Lin, Lau Yen Hui, Yeo Wee Chuan, “Using Analytical Detection Technologies in Singapore”, Institute of Medicine meeting, US Academy of Sciences, 11 March 2012, Washington DC, USA.
6 Yong Yuk Lin, Lau Yen Hui, Lim Thiam Bon, Yang Chiew Yung, Lim Chin Chin, “Applying an Integrated Forensic Methodology in the Examination of Counterfeit Medical Products and Their Packaging”, 6th European Academy of Forensic Science Conference, 20–24 August 2012, Netherlands.
7 Lim Chin Chin, Yong Yuk Lin, Yeo Wee Chuan, Lau Yen Hui, “Analytical Techniques to Detect and Fingerprint Unregistered APIs” (2012), DIA Annual China Conference, 21 May 2012, Beijing, China.
8 “Unknown Chemicals and Materials – A Primer for Lawyers”, Singapore Law Gazette, March 2016.
9 https://www.nuh.com.sg/news/media-articles_2213.html
10 “7 charged for sale of bogus contact lenses”, AsiaOne (21 February 2012).
11 Public Prosecutor v Koh Peng Kiat and another appeal [2014] 4 SLR 703.
12 “INTERPOL applauds Southeast Asia Operation Storm II’s success in disrupting trade of counterfeit medical products”, 27 January 2010 – Media Release.
